Introduction
In a saturation binding experiment, you vary the concentration of radioligand and measure binding. The goal is to determine the Kd (ligand concentration that binds to half the receptor sites at equilibrium) and Bmax (maximum number of binding sites).
The ligand binds not only to receptors sites, but also to nonspecific sites. There are three approaches to dealing with nonspecific binding.
•Subtract off the nonspecific, and analyze only the specific binding.
•Analyze the total binding only, inferring the amount of nonspecific binding from the shape of the total binding curve. Learn more.
•Globally analyze the total and nonspecific binding at one time. This is the best approach, and the details are explained below.
Step by step
Create an XY data table. Enter radioligand concentration into X, total binding into Y, and nonspecific binding into column B.
Use any convenient units for X. The Kd will be reported in those same concentration units. Use the same units for total and nonspecific binding. The Bmax will be reported in those same units.
Alternatively choose the sample data set: Binding - Saturation binding to total and nonspecific.
From the data table, click Analyze, choose nonlinear regression, choose the panel of Saturation Binding equations, and choose One site -- Total and nonspecific binding.
Consider constraining the parameter Background to a constant value of zero. This is the measured 'binding' when there is no radioligand binding added, so represents the counter background, if there is any.
Model
specific=Bmax*X/(X+Kd)
nonspecific=NS*X + Background
<A>Y=specific+nonspecific
<B>Y=nonspecific
The <A> and <B> syntax means that the third line is only used for data set A (total binding) while the fourth line is used only for data set B (nonspecific).
The parameters NS and Background are shared between the two data sets.
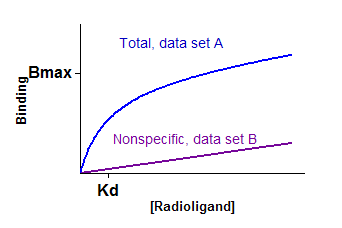
Interpret the parameters
Bmax is the maximum specific binding in the same units as Y. It is the specific binding extrapolated to very high concentrations of radioligand, so it value is is almost always higher than any specific binding measured in your experiment.
Kd is the equilibrium dissociation constant, in the same units as X. It is the radioligand concentration needed to achieve a half-maximum binding at equilibrium.
NS is the slope of nonspecific binding in Y units divided by X units.
Background is the amount of nonspecific binding with no added radioligand. This represents counter background. If your counter automatically subtracts off the background signal, you can constrain Background to a constant value of zero.